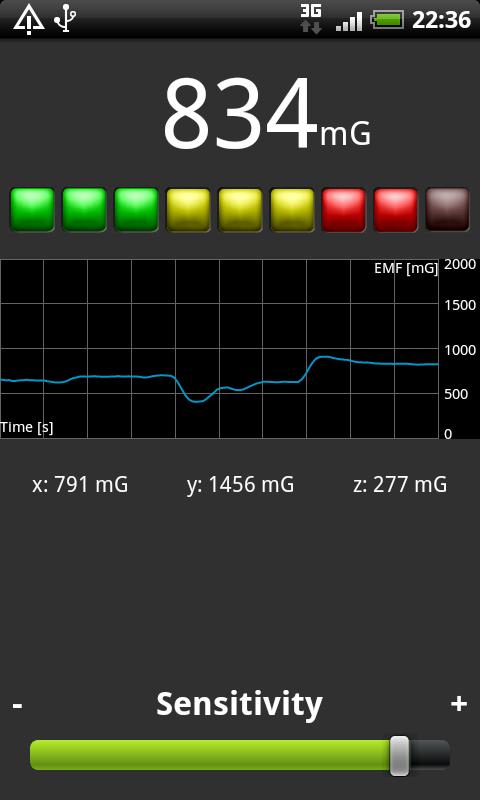
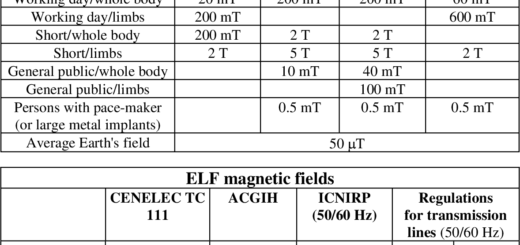
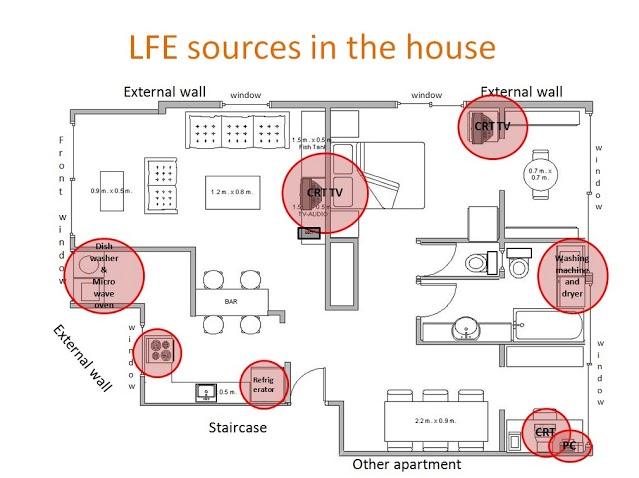
Pacemaker EMI Testing / Biomedical Implant Electromagnetic Interference EMI Surveys – EMF Sources & Issues

214.912.4691 Commercial Clients ONLY
ScanTech DOES NOT perform residential testing OR consulting for homeowners / tenants – all services are COMMERCIAL ONLY
HOMEOWNERS & RESIDENTIAL CLIENTS CLICK HERE
Q. What kinds of fields can cause Electromagnetic Interference with a pacemaker, CRT-D, ICD or other biomedical implants and how do you determine EMI on a pacemaker?
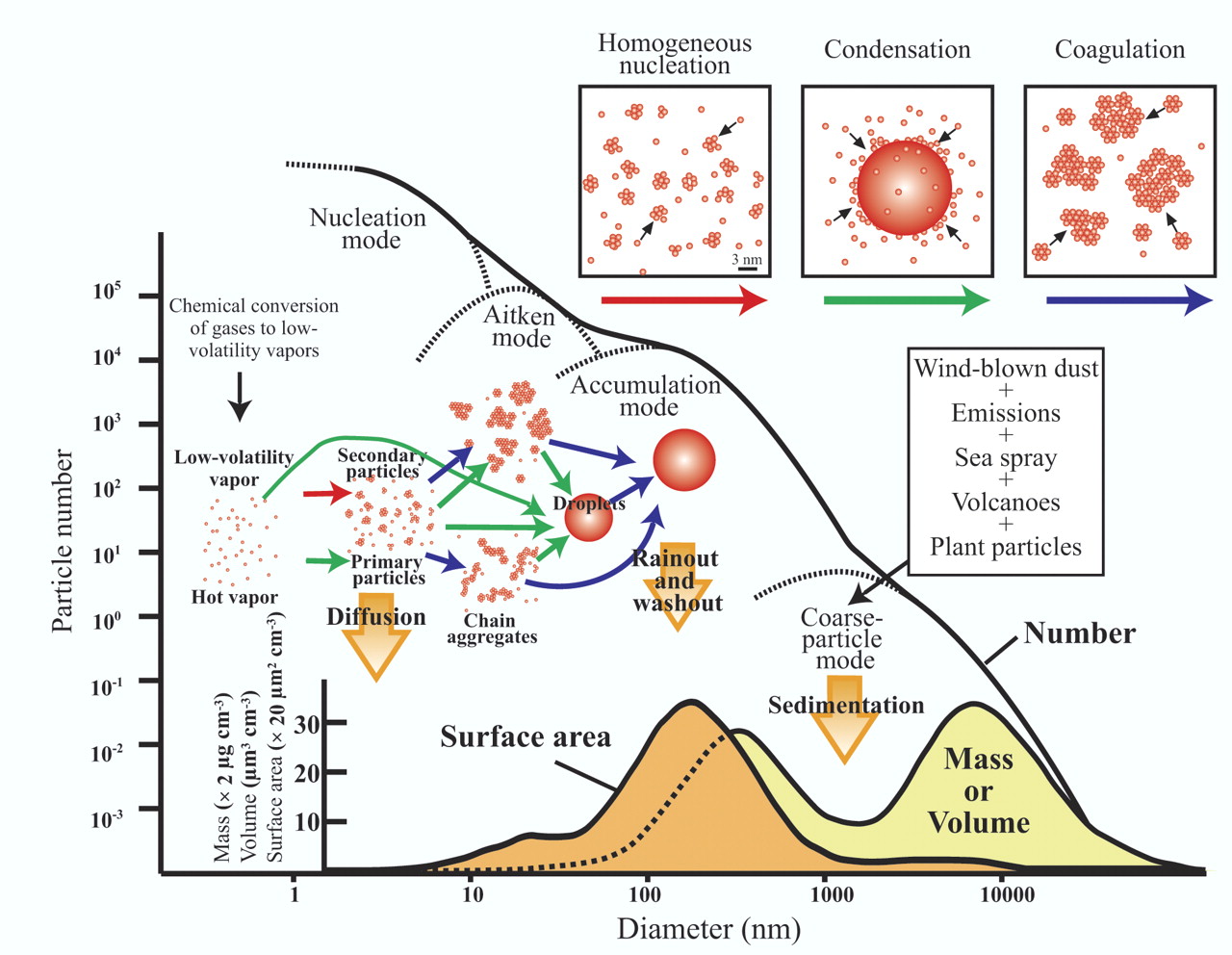
While pacemakers and other biomedical implants and implantable devices are somewhat more resistant to electromagnetic interference (EMI) than in previous generations, there are also more sources of potential EMI from new technologies such as hybrid / electric vehicles, wireless chargers and so forth.
For example, the DC magnetic field from headphones can exceed 10 Gauss and can demonstrably interfere with the operation of a pacemaker. Cardiac centers a few years ago did a study and found that up to 15 % of patients with a pacemaker experienced interference issues when headphone came within 1.2 inches of the device, and up to 30 % of patients with an ICD (Implanted Cardioverter Defibrillator) also demonstrated operational abnormalities caused by the close proximity of the speaker.
While cell phones and MP3 players are less likely to cause issues, they should still be kept away from the heart / bioimplant area as there is a chance that RF energy from these electronic device could cause unpredictable behavior. Several years ago, there were some studies that indicated that MP3 players themselves (separate from the magnetic field of the speakers) could not affect a pacemaker, but since then MP3 players have evolved to other transmission modalities which involve using radiated Bluetooth frequencies.
Also, studies (June 2015) have shown that cell phone can influence pacemakers in unexpected ways. At close range (within 6 inches) pacemakers can misinterpret the signal from a cell phone as a cardiac signal which then responds by consequently pausing the cardiac rhythm of the patient and could lead to fainting. For ICDs, the cell phone signal could be mistaken for ventricular tachyarrhythmia and lead to a painful shock as the ICD is programmed to respond to what it thinks is abnormal heart rhythm.
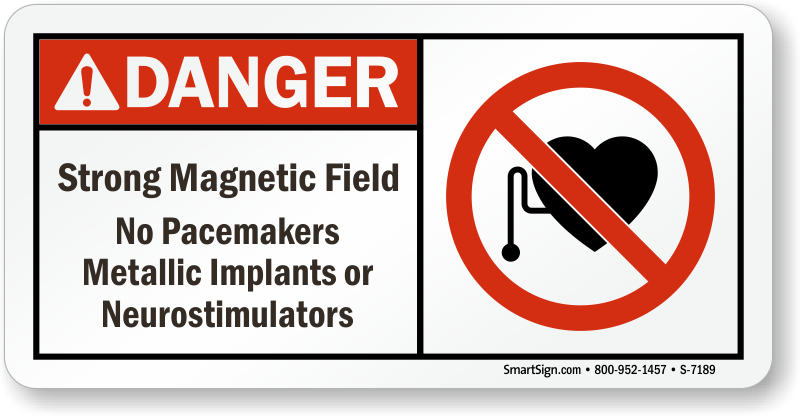
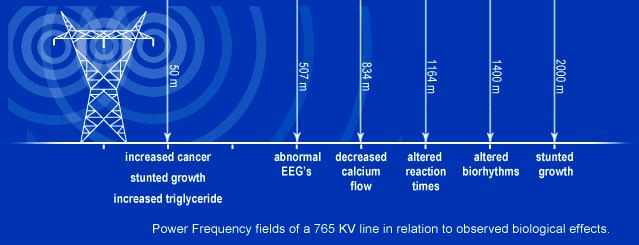
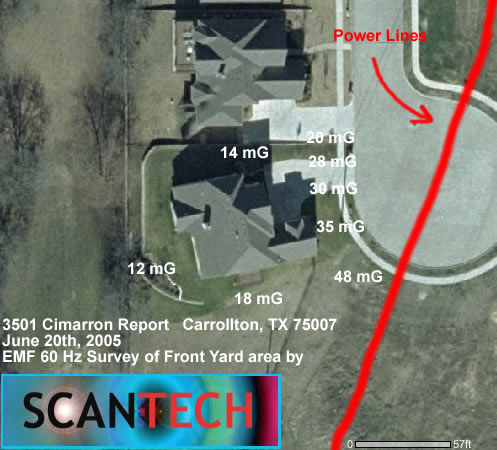
Also, high electric fields such as those beneath high voltage power lines could induce similar behavior if the implants are set to configurations which lower their EMI susceptibility.
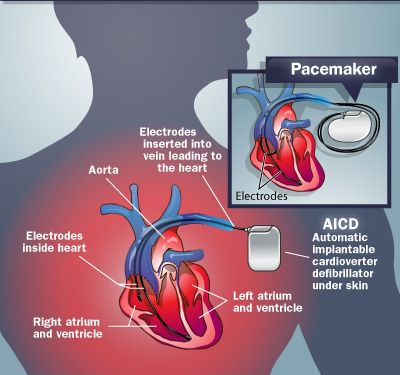
Pacemaker ICD Diagram
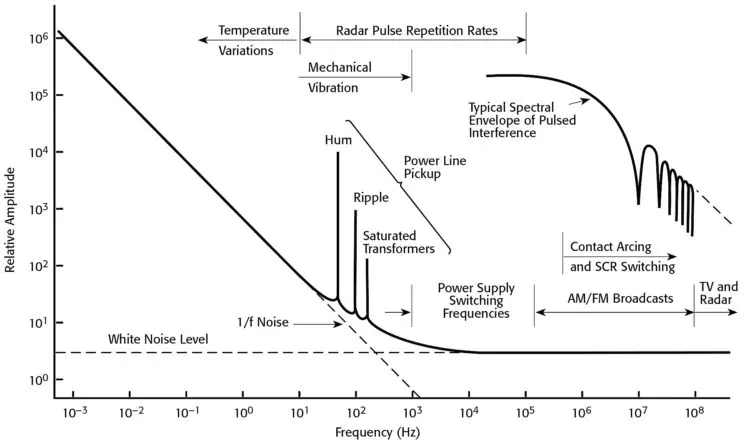
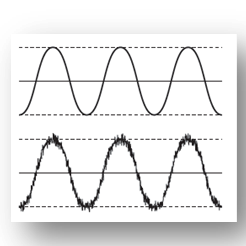
Electromagnetic Interference EMI Testing Voltage Noise Frequency Spectrum
Pacemaker & Implanted Biomedical Device EMF / EMI Testing and Compliance Surveys for Employee / Patient Safety
We offer pacemaker and implantable Medical Device EMI Compliance Testing (Pacemakers, Implantable Defibrillators, ICD’s etc. – Medtronics, St. Jude Medical, Boston Scientific, etc.) for employees looking to return to work after implant surgery to identify potential hazards from AC/DC Magnetic and Electric Fields, Microwaves, RF and general EMI (Electromagnetic Interference). Medical Device / Medical Imaging EMI (Electromagnetic Interference) Testing for safety compliance specs (Pacemakers, ICDs, Implantable Defibrillators, PA Pulmonary Artery HF Sensors, Spinal Cord, Neural and Neuromodulation Implants, SCS, SNS, VNS, EKG, ECG, EEG, EMG, ambulatory Holter Units, insulin infusion pumps and other diagnostic biomedical equipment issues with MRI and ultrasound imaging.
As more technologies such as WiFi, Wireless Internet, Bluetooth, RFID and other communication linked technologies began to saturate offices, industrial areas and manufacturing plants, the potential for EMI interference conflicts only increases. As an experienced EMF consulting company with over 22 year of experience, ScanTech can help minimize safety risks from pacemaker EMI by quickly identifying EMF sources and giving a detailed EMF site survey report.
While newer generation designs tend to be more resistant to external influences, I still routinely find sources of EMF and EMI Electromagnetic interference that exceed the manufacturer specifications, particularly in work and industrial environments. (but residences are not exempt for reasons discussed below)
This is due to a number of reasons:
1) Our electromagnetic spectrum is more crowded than ever and we are constantly adding new electronic devices and spectral intensity to our everyday environment. Examples include:
- Wireless phone chargers
- Battery chargers for electric vehicles & power tools
- WiFi, Wireless Internet and Bluetooth
- Hybrid Electric Vehicles (HEVs) and Fully Electric Vehicles (EVs)
- Electric commuter rail systems
- Wireless microphones and media systems
- Smartphones – cell phones, cordless phones (particularly those with inductive charging)
- Security scanners for weapons detection
- RFID (Radio Frequency Identification) reader systems for entry control
- Portable music devices, Bluetooth Speakers, headphones for iPods and MP3 players
- Laptops, tablets, desktop computer power supplies
- Electric scooters, drones, hybrid & electric vehicles including scooters, utility vehicles, golf carts, Segways, Gators, Mules, etc.
- Internet access points, wireless repeaters, amplifier boosters and cellular phone boosters (AWS or Advanced Wireless Systems in the 1.7 GHz range)
2) Many industrial and manufacturing environments have equipment which emits and broadcasts EMFs at relatively high power levels over a broad range of frequencies:
- Electric welding equipment (MIG, TIG, SMAW, etc.) and welders
- Corded and cordless power tools
- Generators, alternators, fan & cooling motors
- RF clam shell sealers
- Semiconductor fabrication plant equipment assets
- UPS (Uninterruptible Power Supply) banks & DC battery backups
- Cleanrooms / semiconductor fabrication equipment
- MCC (Motor Control Centers) Switchgear Rooms and VFD (Variable Frequency Drive) motor drives for AC / DC
- Plant’s backbone electrical distribution system is typically the Medium Voltage 4160 V system
- AC and DC motors found in blower motors / fans, HVAC equipment, pumps (particularly 480 V three phase)
- Cable trays / racks and open (no metal conduit) systems with high amperage power feeds
- Walkie-talkies, base stations & 2 Way Radio Microphones (Motorola & Kenwood in particular)
- Apple iPhones and other phones that use Inductive Charging technology
- Induction furnaces including EAF (Electric Arc Furnace) both AC & DC current
- High powered DC Rectifier arrays associated with battery rooms and electrochemical plants
- Transformers, medium & high voltage power lines & substations
- Industrial electromagnets & jig fixture permanent magnets
- High powered industrial CO2 (Carbon Dioxide) and YAG fiber optic lasers in the kW (kiloWatt) range
- Magnetized structural beams
- Electrochemical manufacturing cell rooms and buildings
BIOMEDICAL IMPLANT SURVEY LINKS
FDA MAUDE (Manufacturer User Device Experience Database
Boston Scientific Technical Support Lines
IEC 61000-4-8 AC Magnetic Field Standards
Agilent IEC 61000-4-8 AC Magnetic Field Specs – PDF Format
Manuals for St. Jude Medical / Abbot Laboratories Biomedical Device Implants
Medtronic Biomedical EMI Testing – PDF Format
Boston Scientific Electromagnetic Compatibility Guide.pdf
Spinal Cord Stimulators (SCS) & MRI Questions
CardioMEMS PA HF EMI Testing Manual – PDF Format
Environmental Electromagnetic EMI Interference Pacemakers ICDs – PDF Format
Implantable Biomedical Devices Pacemakers EMI Testing Electromagnetic Environment – PDF Format
Electromagnetic Interference EMI Pacemakers ICDs Technical Paper- PDF Format
ICNIRP EMF Survey Guidelines – PDF Format
EMI Pacemakers Magnetic Electric Interference – PDF Format
EKG Measurements Noise EMI Electromagnetic Noise PDF Format
WHO IS A CERTIFIED EMF CONSULTANT QUALIFIED TO PERFORM PACEMAKSER EMI TESTING NEAR ME?
ScanTech Technical Consulting is owned and operated by Joel-Anthony Gray who has over 22 years of experience as an EMF consultant, EMI troubleshooting expert and nuclear radiation testing consultant for a variety of commercial and industrial customers. While there is currently no such thing as a Certified EMF Consultant as far as the United States industrial and scientific community at large is concerned, what ScanTech offers is extensive education, experience and multiple certifications in non-ionizing radiation, industrial safety and hygiene. (listed here EMF Credentials)He holds numerous degrees and certifications which qualify him for this title. Call 214.912.4691
WHO DOES ELECTROMAGNETIC FIELD TESTING NEAR ME?
ScanTech Technical Consulting professional commercial electromagnetic field testing and radio frequency testing in the Dallas – Fort Worth Texas area to detect and measure exposures and health risks (if any) to electromagnetic fields and radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES EMF TESTING NEAR ME?
ScanTech Technical Consulting professional commercial electromagnetic field testing and radio frequency testing in the Dallas – Fort Worth Texas area to detect and measure exposures and health risks (if any) to electromagnetic fields and radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES RADIO FREQUENCY (RF) RADIATION TESTING NEAR ME?
ScanTech Technical Consulting provides commercial radio frequency (RF) testing in the Dallas – Fort Worth Texas area to detect and measure exposures to all forms and frequencies of radio frequency radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES PACEMAKER EMI TESTING AND ELECTRICAL INTERFERENCE SURVEYS FOR IMPLANTED BIOMEDICAL DEVICES NEAR ME?
ScanTech Technical Consulting provides professional EMI testing for pacemakers and other biomedical implants throughout the United States to detect and measure exposures to all forms of interference from electromagnetic radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES RF RADIO FREQUENCY CELL TOWER TESTING NEAR ME?
ScanTech Technical Consulting provides commercial cellular testing in the Dallas – Fort Worth Texas area to detect and measure exposures to all forms of RF. Call 214.912.4691
WHO OR WHAT COMPANY DOES ELECTROMAGNETIC RADIATION TESTING NEAR ME?
ScanTech Technical Consulting provides commercial radio frequency (RF) testing in the Dallas – Fort Worth Texas area to detect and measure exposures to all forms of electromagnetic radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES ELECTRICAL INTERFERENCE TESTING NEAR ME?
ScanTech Technical Consulting provides professional radio frequency (RF) testing in the Dallas – Fort Worth Texas area to detect and measure exposures to all forms of electromagnetic radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES RADIOACTIVITY TESTING NEAR ME?
ScanTech Technical Consulting provides professional radioactivity testing in the Dallas – Fort Worth, Houston, Austin and San Antonio areas of Texas to detect and measure exposure to ionizing, nuclear or atomic radiation. Call 214.912.4691
WHO OR WHAT COMPANY DOES DALLAS AREA PHOTOMETRIC LIGHTING SURVEYS OR EXTERIOR LIGHTING STUDIES NEAR ME?
ScanTech Technical Consulting provides professional photometric testing in the Dallas Texas area for commercial clients to help pass lighting ordinances for their Certificate of Occupancy. Call 214.912.4691
WHO OR WHAT COMPANY DOES ESD / ELECTROSTATIC DISCHARGE TESTING NEAR ME?
ScanTech Technical Consulting provides ESD testing in the Dallas – Fort Worth, Austin, San Antonio and Houston Texas area to evaluate, measure and perform Electrostatic Discharge testing and consulting for sensitive environments. Call 214.912.4691
WHO OR WHAT COMPANY DOES INDOOR AIR QUALITY TESTING NEAR ME?
ScanTech Technical Consulting provides certified indoor air quality testing for commercial clients in the Dallas – Fort Worth Texas area detect and measure harmful contaminants. Call 214.912.4691
WHO OR WHAT COMPANY DOES DALLAS GREEN BUILDING INDOOR AIR QUALITY CLEARANCE TESTING NEAR ME?
ScanTech Technical Consulting provides certified indoor air quality testing in the Dallas Texas area for commercial clients to help pass the Dallas Green Building Clearance Code 804.2 for a Certificate of Occupancy. Call 214.912.4691
We often serve clients in Texas, Michigan and Iowa. Cities for onsite commercial testing and inspection services include: Plano, Highland Park, University Park, Park Cities, Las Colinas, Arlington, Fort Worth, Houston, Austin, San Antonio, Shreveport, Grapevine, Frisco, Denton, McKinney, Allen, Lewisville, Irving, Mesquite, Bedford, Euless, Richardson, Coppell, Grand Prairie, Garland, Addison, Farmers Branch, Rockwall, Carrollton, Parker, Rowlett, Lucas, Fairview, Park Cities, Keller, Roanoke, The Colony, Highland Village, Lake Dallas, Corinth, Prosper, Duncanville, Lancaster, Rowlett, Royse City, Princeton, Trophy Club, Southlake, Hurst, Round Rock, Georgetown, San Marcos, Cedar Park, The Woodlands and Spring. Counties served include Dallas, Collin, Denton, Tarrant, Rockwall, Harris and Travis County.
LARGER COMMERCIAL PROJECT SERVICE RANGE – NATIONAL & INTERNATIONAL
Alabama | Alaska | Arizona | Arkansas | California | Colorado | Connecticut | Delaware | Florida | Georgia | Hawaii | Idaho | Illinois | Indiana | Iowa | Kansas | Kentucky | Louisiana | Maine | Maryland | Massachusetts | Michigan | Minnesota | Mississippi | Missouri | Montana | Nebraska | Nevada | New Hampshire | New Jersey | New Mexico | New York | North Carolina | North Dakota | Ohio | Oklahoma | Oregon | Pennsylvania | Rhode Island | South Carolina | South Dakota | Tennessee | Texas | Utah | Vermont | Virginia | Washington | West Virginia | Wisconsin | Wyoming | Washington D.C. (District of Columbia)
Major US Cities: Chicago, Detroit, Phoenix, Denver, Salt Lake City, Miami, Grand Rapids, Lansing, Nashville, Memphis, Atlanta, Charleston, Raleigh-Durham, Charlotte, Des Moines, Milwaukee
Countries served include the United States, Canada, Australia, New Zealand the UK / United Kingdom (England, Scotland, Wales, Ireland) and Western Europe.
*LEGAL NOTICE*
All information on this website either written or implied is the express opinion of ScanTech Technical Consulting. ScanTech Technical Consulting and it’s owners are not responsible or liable for any damages arising from the misuse, misinterpretation or other consequences of the content of this website either in part or in whole. This includes all external weblinks, PDF documents, photos or other references (informational or otherwise) to 3rd parties including government agencies, health organizations, etc.
15770 Dallas Pkwy Suite # 900 Dallas, TX 75248 (Not Accepting Visitors due to C19)
Phone: (214).912.4691 https://emfsurvey.com
ScanTech Technical Consulting: Professional Electromagnetic EMF Consulting / EMI Testing for Biomedical Implant & Equipment, Indoor Air Quality (IAQ) Testing & Environmental Studies and Inspections
COPYRIGHT 2002 – 2024