Radon Safety Inspections: More Interesting Facts You Didn’t Know
Some of you are aware from reading my blog or elsewhere that radon gas exposure is estimated to cause 15,000 – 20,000 deaths in the United States but is even more likely to affect smokers.
But how much more?
As of this writing it is estimate that radon is responsible for 3-5 % of all fatal lung cancers, but specifically up to 10-15 % for smokers.
In the chart below, you can see that radon gas contributes the majority (over 1/2) of the harmful radiation that an average person is exposed to over their lifetime including medical X-Rays.
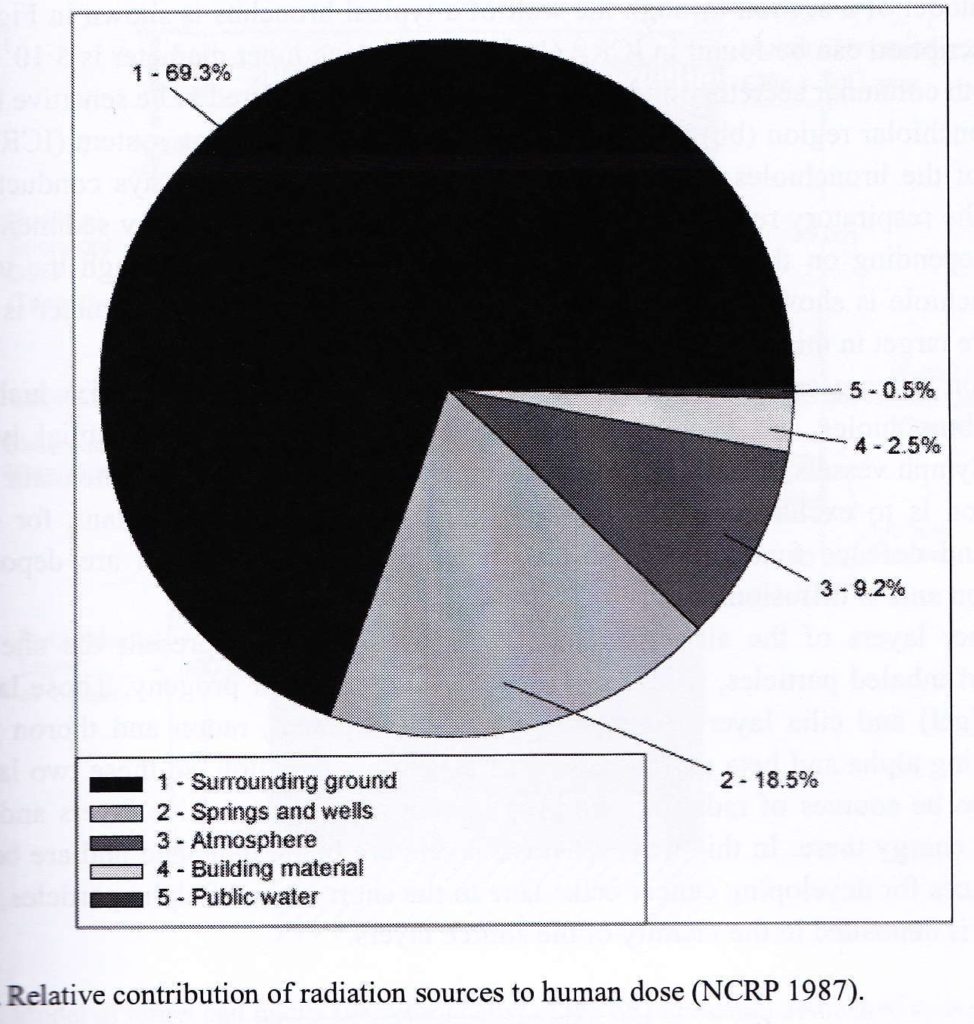
Radon Safety – Indoor Dwellings Contribution Pie Chart
Where does the radon gas come from?
Much of it is from soil exposure, but a sizable contribution within indoor dwellings comes from building materials such as brick, concrete, etc. and particularly stone such as mildly radioactive granite and marble which are found in kitchen and bath counter-tops. (ScanTech can test natural stone materials for excess radioactivity) Other potential sources are in the water and natural gas supplies; even in burning coal.
Radon is soluble in water, particularly cold water and can be released while cooking, bathing and cleaning. It can also find it’s way into the breathable atmosphere through commercial activities such as ore processing, burning of coal in power stations and the use of agricultural fertilizers.
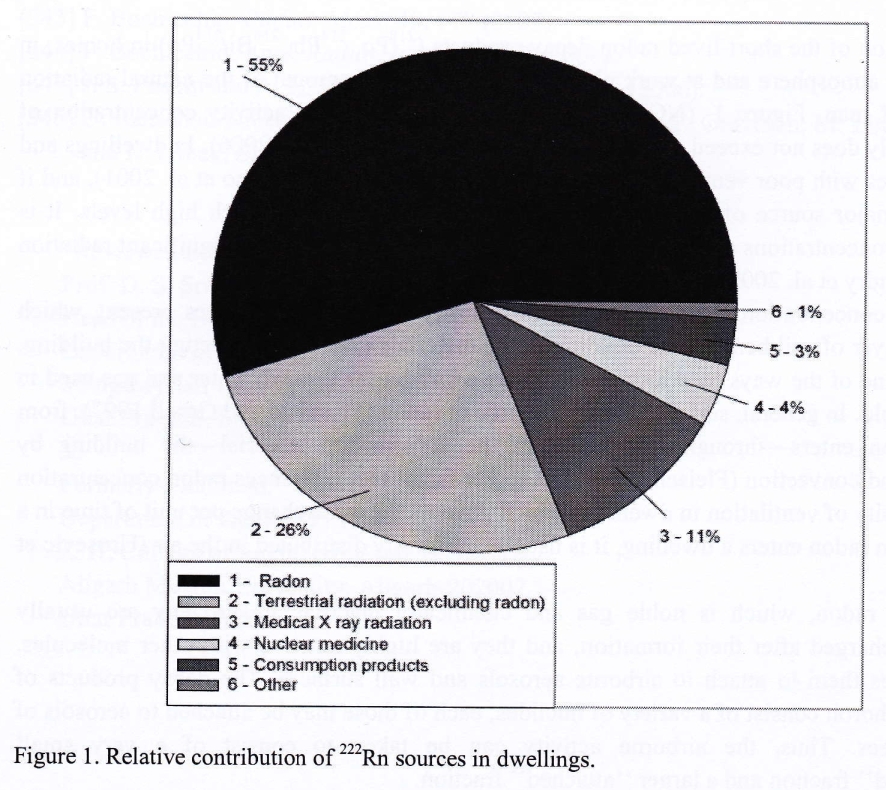
Radon Gas Overall Contribution Chart
One interesting property of note with radon is that is has a rather strong diffusion ability, which means that it spreads out and infiltrates air and water easier than many other substances. The official diffusion coefficient is 0.12 cm^2/sec in air and 1.37 * 10^-5 cm^2 in water at 25 C. (roughly room temperature)
The solubility coefficient is the measure of gas solubility in water. It is defined as the ratio of the radon concentration in water to that in air. At 20 C, the solubility coefficient is 0.25 which means that radon is distributed preferentially in air rather than in water. (a ratio of 4:1) The radon solubility is 510 cm^3/L at 0 C as the solubility of radon goes up in colder water. (this is rather the opposite of most other chemical substances where the solubility tends to go up the warmer the water)